Abstract
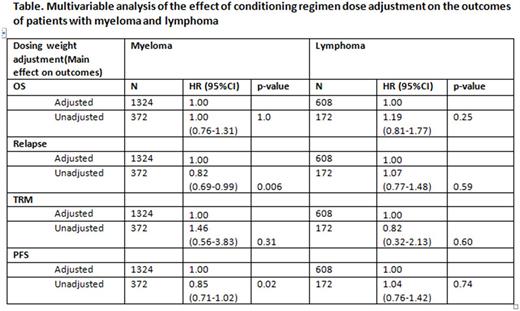
There are limited data on whether to adjust high-dose chemotherapy prior to autologous transplant in overweight patients. We performed a registry-based retrospective analysis on the effect of dose adjustment on the outcomes of patients with body mass index (BMI) ≥ 30 kg/m2. Dose adjustment was defined as a reduction in standard dosing of ≥ 20%. We studied autologous transplant recipients with myeloma (n=1696) treated with high-dose melphalan and lymphomas (Hodgkin/non-Hodgkin; n=781) who received carmustine, etoposide, cytarabine, and melphalan (BEAM) conditioning, respectively. In the myeloma group, chemotherapy dose was adjusted in 1324 (78%). Age, sex, BMI, race, Karnofsky performance score (KPS), co-morbidity score (HCT-CI), and disease features (stage at diagnosis, disease status and time to transplant) were similar between adjusted and unadjusted dose groups. In the multivariate analysis (Table) for myeloma patients, adjusting (reducing) the melphalan dose was not an independent predictor of survival and treatment-related mortality (TRM), whereas there was a reduction in relapse and improvement in progression-free survival in myeloma patients who received an unadjusted chemotherapy dose. In the myeloma group, younger age, early disease stage at diagnosis, higher KPS, and chemotherapy sensitive disease were independent predictors of superior survival. In the lymphoma cohort, chemotherapy dose was adjusted in 608 (78%). There was a similar distribution of age, sex, BMI, race, KPS, and HCT-CI between patients with dose adjusted and unadjusted groups. In addition, the distribution of lymphoma subtypes, chemotherapy sensitivity, time from diagnosis to transplant, and year of transplant were similar between dose adjusted and unadjusted groups. In the multivariate analysis (Table) for lymphoma patients, adjusting the conditioning regimen chemotherapy dose did not affect survival, PFS, relapse or TRM. In the lymphoma group, younger age and higher KPS were independent predictors of superior survival. There found center effect on the outcomes of both myeloma and lymphoma groups. Our study demonstrates that adjusting (reducing) the conditioning regimen chemotherapy dose for autologous transplant patients with BMI ≥ 30 with myeloma receiving melphalan and lymphoma receiving BEAM did not influence survival. Myeloma patients receiving unadjusted, and thus higher dose chemotherapy, experienced less early relapse. In summary, our data suggest that patients with high BMI undergoing autologous transplantation with melphalan or BEAM do not require dose adjustment.
Brunstein: Novartis: Research Funding; Magenta Therapeutics: Research Funding. De Lima: Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Research Funding. Savani: Jazz Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal